The CCPN project contain the following data:
Before all, you will need to create a new project.

ARIAweb will thus read the content of the CCPN project archive and will be ready for preparing a new structure calculation.
Now that you have created your ARIAweb project and uploaded your CCPN archive, you can prepare your ARIA structure calculation.
Click the button under Structure calculation (Figure 2).
You now have a new form where you will be able to validate or modify all the parameters for your ARIA run. The Structure Calculation form is designed to guide the user through the main categories of parameters that need to be checked (if default values are used) or specified (if a user wants to change with customized values). The 4 main categories of parameters are:
Start by giving a name to this ARIA job, e.g. hrdc.
Click the button to move to the next section of the form.
In the DATA section, you will see that the Molecular system is already pre-filled with the sequence defined in the CCPN project (hrdc:A) and the format is set to CCPN.
Click to move to the Spectra definitions.
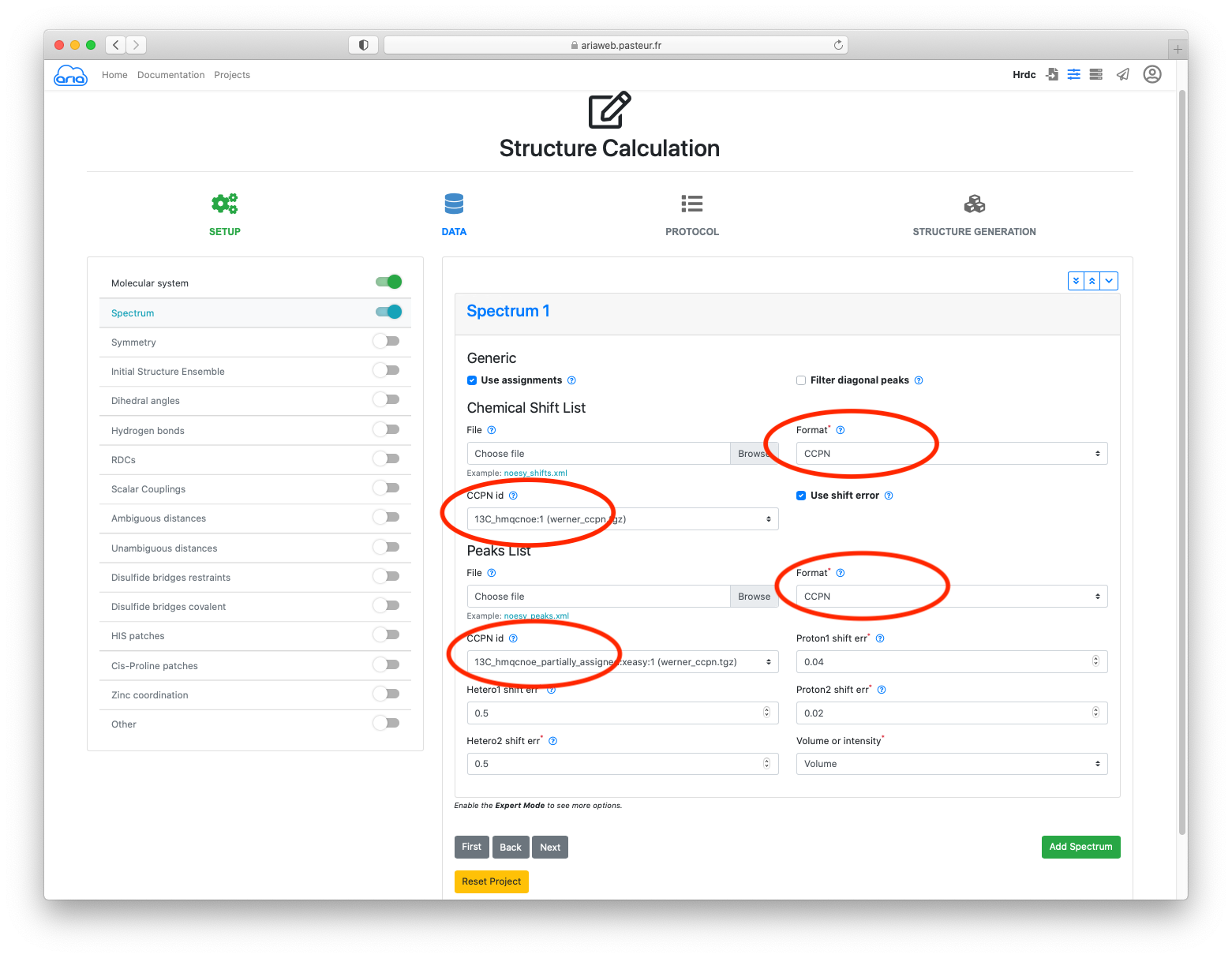
There are 2 spectra (or peak-lists in the CCPN) project.
For the 1st spectrum (13C-resolved NOESY), specify the CCPN format for the Chemical shift list. Select the 13C_hmqcnoe:1 list under CCPN id
Now for the Peaks List, specify again the CCPN and select the 13Chmqcnoepartially_assigned:xeasy:1 list under CCPN id (See Figure below)
Leave all parameters as default (such as the assignment tolerances for the 3 dimensions of the NOE peak-lists).
Click button at the botton of the page to add a second spectrum items.
Do the same for the 15N-resolved NOESY
In addition to NOE data, we will use hydrogen bonds restraints (for slow-exchanging amide protons) and dihedral angle restraint (derived from 3J(HN,Ha) coupling constants).
To do so, activate the sliders on the left for Dihedral angles and Hydrogen bonds (Figure 5).
Click the button to move to Dihedral angles.
Select CCPN as format and the 1:2:dihedrals as CCPN id (Figure 5).
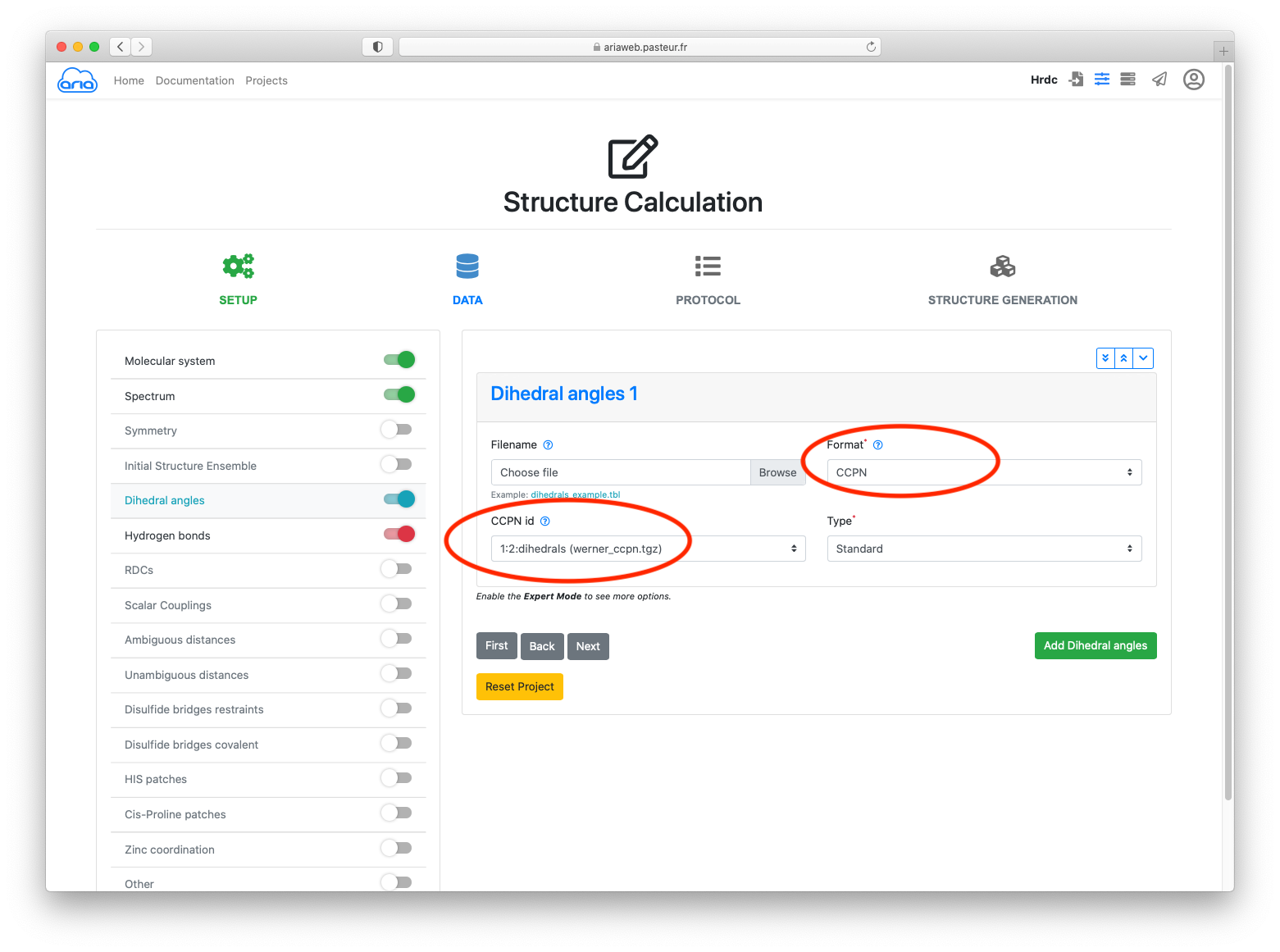
Click the button to move to Hydrogen bonds.
Select CCPN as format and the 1:1:HBonds as CCPN id for the hydrogen bond restraints.
Finally, click the button again.
For the practical, we use all parameters as default for PROCOL and STRUCTURE GENERATION. Continue clicking until you see the button.
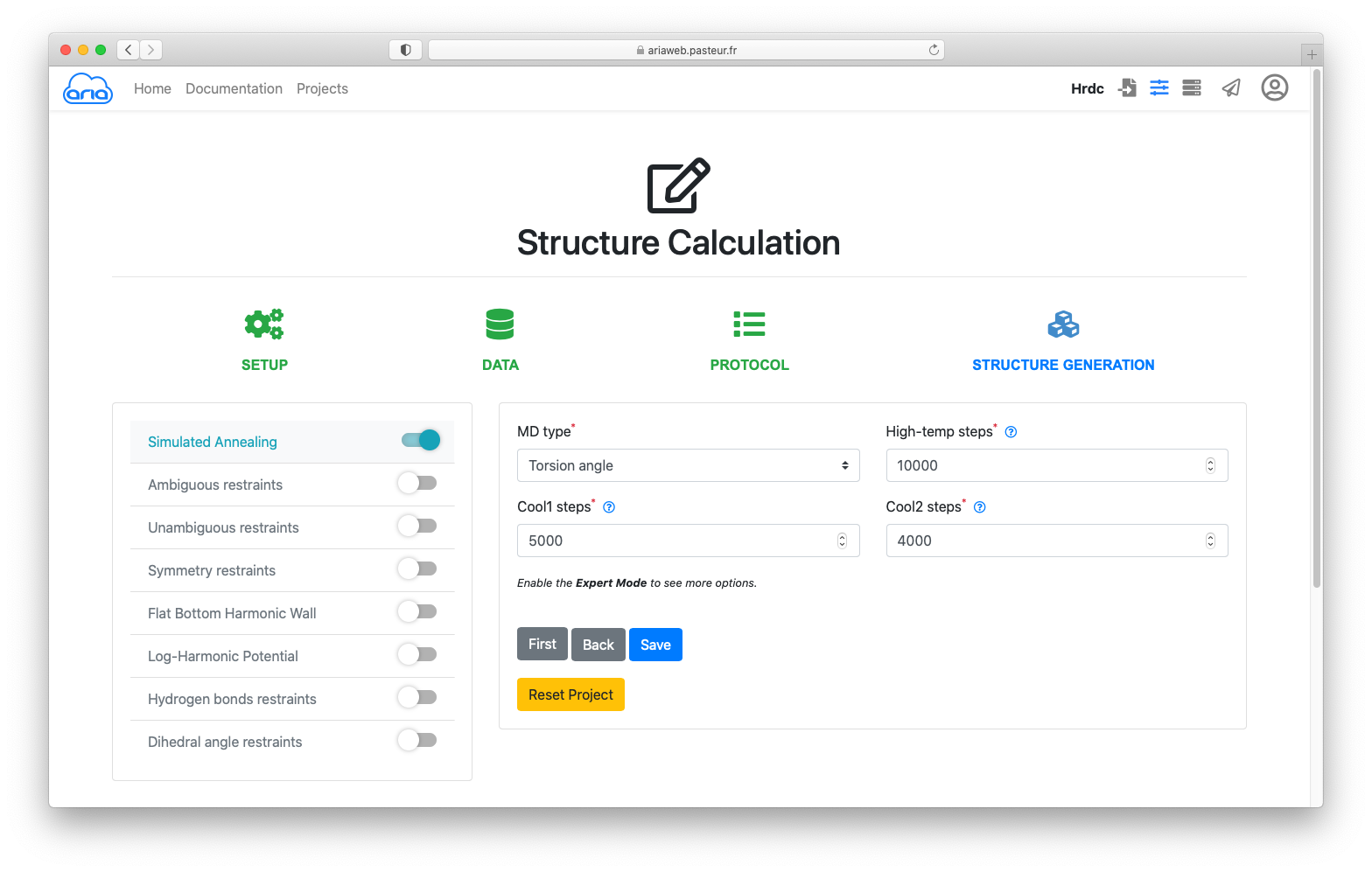
Clicking will save (no surprise here) your parameters and you will be directed to the page where can submit the ARIA calculation job.
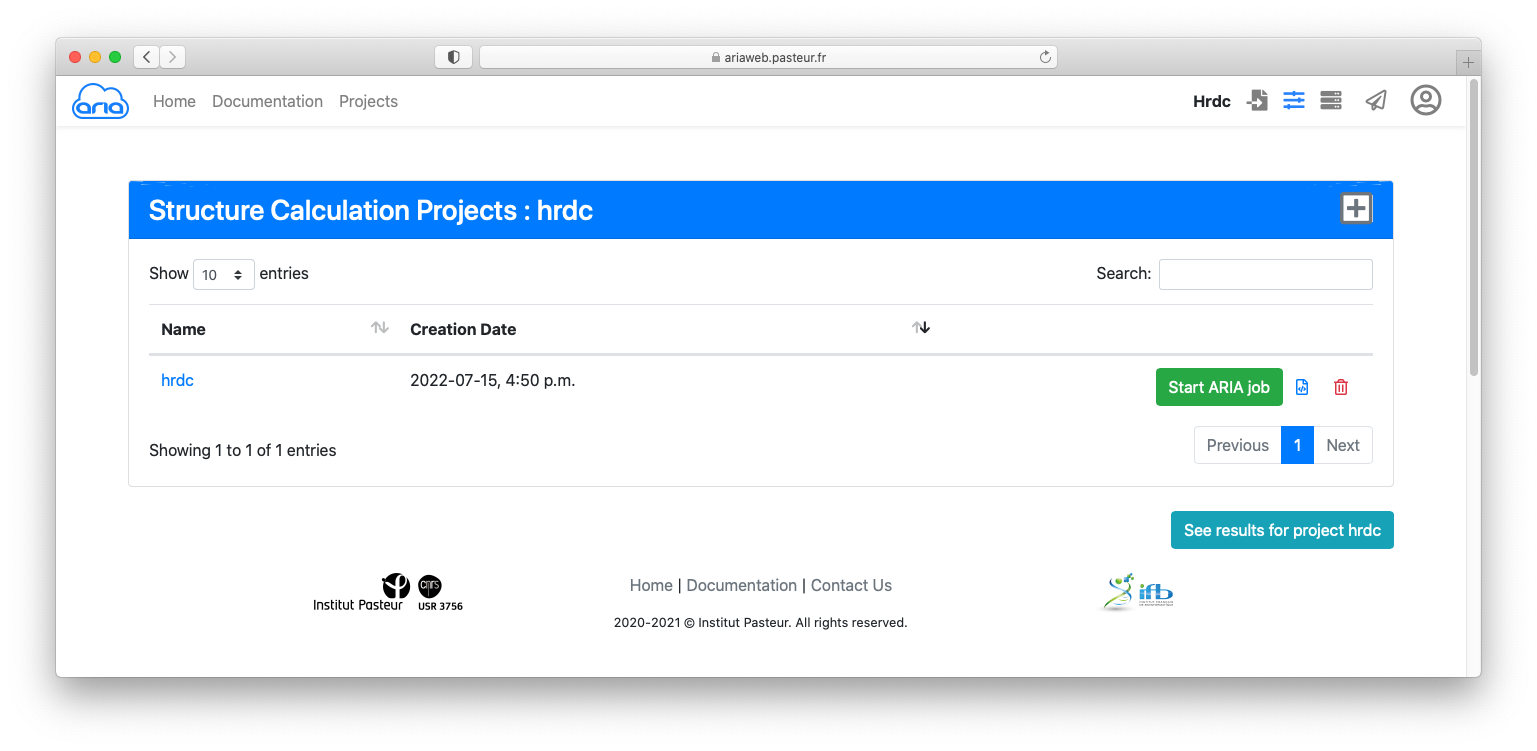
Click the button to submit you structure calculation. You will be redirected to a Results page where you can see the status of your ARIA job. See here for an explanation on the status icon.
Depending on the server load the calculation might take ~30 minutes to hours... For the rest of the practical you will not have to wait until the calculation is finished. We have pre-calculated results that will allow you to visualise and analyse the results of an ARIA job.
If your job is finished (status icon ), click on the button to start the job analysis and visualize the results.
Results archive can be downloaded using .
The visualisation page allows to view an analysed the results of a structure calculation job. Final structure ensemble generated by ARIAweb are displayed interactively, with various representations. Final restraints statistics, structure quality checks and bundle RMSD are shown to help the user interprets the reliability of the results. On top of that, more graphs, restraints validation and PDB files can be downloaded directly (Figure 8).

Results for all ARIA iterations can be shown by selecting an iteration in the Iterations tab. The main component of the Visualisation page is the NGL viewer. By default, several representations of the structure ensemble generated by ARIA are shown:
The Representation tab (below the NGL viewer) allows to change the visibility, styling and coloring of a selected representation. By default, all structures in the bundle are shown; use the filter_frames button to select individual models for display).
The add_circle button allows to upload another PDB file to superimpose on the ARIA structure ensemble (use the layers button to superimpose on a selected structure).
Below, Restraints statistics recapitulate the number of restraints and the trend since the previous iteration for:
The Contribution histogram gives count of restraints (from each input spectra) that have 1 (i.e. unambiguous) or more (i.e. ambiguous) assignment possibilities (or "contributions"). Ultimately, ARIA tries to reduce the ambiguity in NOE assignments, producing more unambiguous restraints. Truly ambiguous assignments can remain due to spectral overlap or chemical shifts degeneracy.
The Ensemble RMSD graph shows, for each iteration, the RMSD of the structure ensemble generated by ARIA ("bundle"), computed as the mean RMSD when superimposing of the ensemble average (after iterative superimposition). Low RMSD (< 1-2 Å) is good indicator of convergence of the NOE assignment and structure calculation process by ARIA.
The Quality checks panel summarizes the results of 3 main structural quality validators: WHAT-IF, Procheck and Molprobity. Quality scores are shown on a slider from bad to good values. Bad quality score values indicate that the input data may contain errors/inconsistencies and that ARIA was not able to produce a high quality model. We provide here some indicators on how to judge the quality checks:
Procheck Ramachandran percentage: for typical NMR structures deposited in the PDB, 80% of the dihedral angles lie within the preferred region of the Ramachandran plot. For high-resolution NMR structures, a higher percentage is expected (90%).
WHAT-IF Z-scores: WHAT-IF results are presented in the form of overall Z-scores. In general, structures with Z-scores between -2 and +2 are considered to be within a normal range and are thus good structures, while structures with Z-scores lower than -2 should be inspected further. Useful indicators of good quality are Backbone conformation and Packing quality. The bump-score also reports the number of van der Waals violations per 100 residues.
WHAT-IF profiles: recently, some studies have stressed that global structural indicators are not sufficient to detect errors in structures and suggested examining parameters on a per-residue basis. Such profiles for the WHAT-IF scores are produced by ARIA in the form of a PDF file. Thus, poor quality regions can be precisely identified.
Molprobity clashscore. this reports the number of overlaps >0.4Å per thousand atoms. For typical NMR structures deposited in the PDB, this score is generally high (>10). From our experience, the application of the log-harmonic potential along with automated weight estimation significantly improves this situation (see the More results panels).
The More results panel provides a link to a standard summary table of structural and restraints statistics (iteration 8 and refine)
Additional results are also accessible:
We invite you to read the following book chapter to learn more about ARIA and on how to judge the quality and reliability of structures determined with ARIA from NMR data.
Bardiaux B, Malliavin T, Nilges M. ARIA for solution and solid-state NMR. Methods Mol Biol. 2012;831:453-83. https://doi.org/10.1007/978-1-61779-480-3_23
Back to top | Home | PDF version
© 2022 Benjamin Bardiaux (Institut Pasteur/CNRS)